Blood-Brain Barrier: Separating Brain from Blood
- Sten de Schrijver

- Dec 5, 2022
- 6 min read
The brain is the most complex and mysterious organ in the body. Because of its importance to the functionality of a person, it is very well protected by the blood-brain barrier, or BBB for short. The BBB is a system of cells that form a very tight lining. It allows for the selective uptake of nutrients, while toxins, or any other particles that could be harmful to the brain, cannot pass. The malfunctioning of the BBB gives rise to less regulated uptake and can result in serious brain damage. This actually happens in a number of diseases that affect the nervous system, including HIV and Alzheimer’s Disease. Meanwhile, despite its intention to protect the brain, the BBB also makes it very difficult to localise medicine into, or near, the brain. This in turn majorly affects treatments for brain diseases (Daneman & Prat, 2015).
Structure and Function of the Blood-Brain Barrier
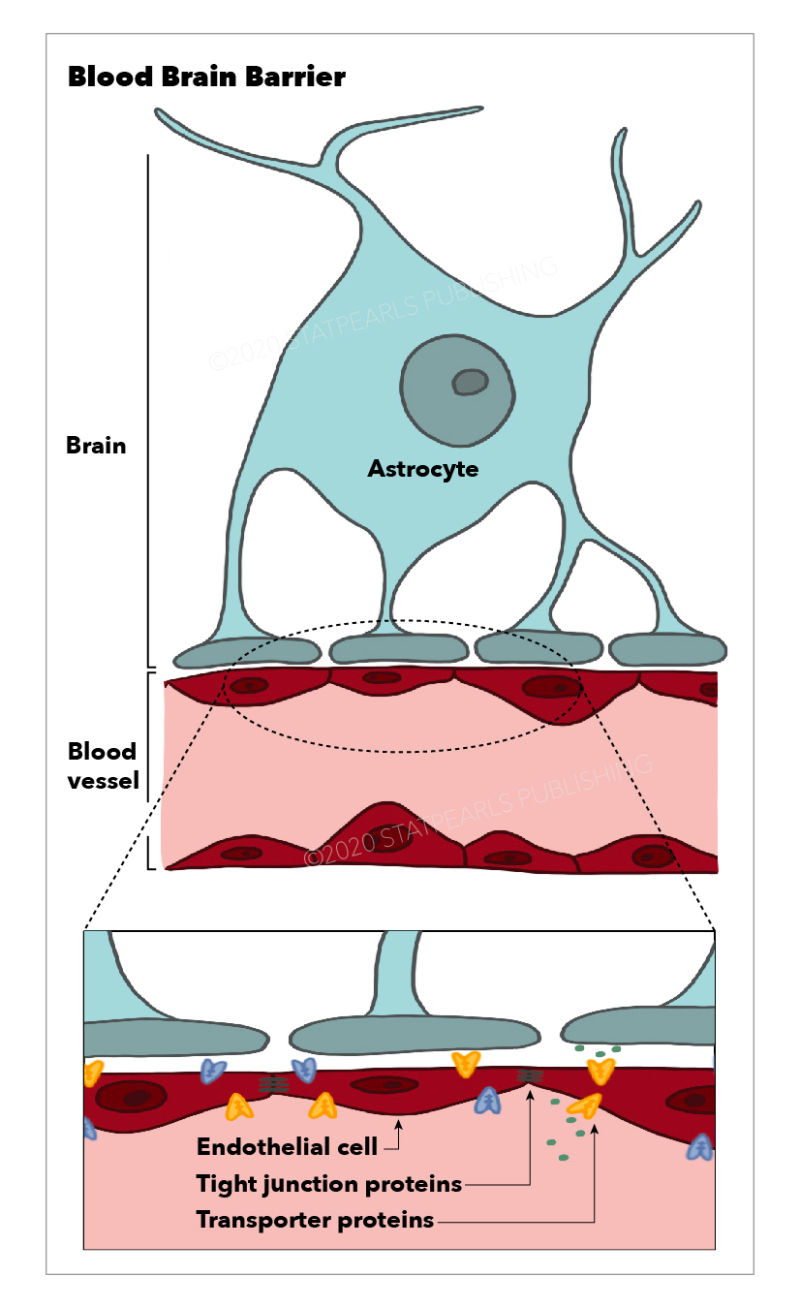
The circulatory system has two goals. First, it allows for the transport of oxygen and nutrients to organs and tissues. Second, it makes sure that whatever a tissue no longer needs is transported away. Arteries are large blood vessels that carry blood away from the heart. At tissue sites, the arteries become smaller, until they form arterioles, and eventually capillaries (Daneman & Prat, 2015). Capillaries are the smallest type of blood vessels, in which the uptake and removal of molecules take place. This is where oxygen is taken up by the tissue, while unwanted carbon dioxide is released into the bloodstream. These capillaries then merge into venules which become veins, large blood vessels that transport oxygen-poor blood toward the heart (Daneman & Prat, 2015). All these blood vessels contain endothelial cells, forming a strong and solid inner lining. Between these cells are gaps that allow for the exchange of molecules. In relative terms, the gaps between endothelial cells are larger when the size of the vessels becomes smaller. This is why most exchange happens in the capillaries, which are small enough to reach virtually anywhere in the human body (Sandoo et al., 2010).

The microvasculature is a collection of arterioles, capillaries, and venules. It is what the BBB is made up of (Daneman & Prat, 2015). The difference between this microvasculature and that of normal tissue is that the endothelial cells are much more tightly packed together. They form a wall by attaching to each other with so-called tight junctions. These junctions are intercellular protein structures formed between neighbouring cells to anchor one another. As cells are brought closer together, the gap between them becomes very small (Cong & Kong, 2020). What is truly unique about endothelial cells in the BBB, though, is that they contain an incredible amount of mitochondria. The mitochondrion is the powerhouse of the cell. Richard Daneman, a researcher at the University of California San Diego and an expert when it comes to the BBB, has once stated that these cells need so much energy for the active transportation of nutrients in and out of the brain (Daneman & Prat, 2015). Normally, there can be passive transport of ions and molecules into cells, but because neurons require such a stable environment, BBB endothelial cells have to do everything in their power to not let a single ion pass the barrier without its active regulation (Daneman & Prat, 2015). Additionally, the cells that form this strict lining contain enzymes that are known to metabolise and break down larger proteins, often drugs (Persidsky et al., 2006).
Homeostasis, which is another word for equilibrium, is the result of a very complex collaboration of endothelial cells, astrocytes, immune cells, and more types of cells (Daneman & Prat, 2015; Persidsky et al., 2006). Mechanisms of homeostasis are far from being fully understood, but are crucial for the proper functioning of the brain. Due to the high sensitivity of the brain, it requires a stable environment to thrive. This homeostasis is achieved by the incredible selection and exchange of particles into and out of the brain, which in turn is governed by the blood-brain barrier. One of the biggest questions about the BBB right now is whether its structure is the same across the surface of the brain. After all, every part of the brain is responsible for very specific tasks, which might require different selective uptake of nutrients depending on the location. As a result, it might be possible that transporter proteins are differentially expressed based on specific regions of the blood-brain barrier (Persidsky et al., 2006).

The hypothesis that the BBB structure is different depending on location is supported by the fact that some parts of the brain do not have a blood-brain barrier. The posterior pituitary, for example, is responsible for the secretion of many hormones, which are relatively large molecules that contain lipids. Because of their size, it would be very difficult for hormones to cross the BBB. This is the reason why BBB is absent in organs like the posterior pituitary, termed circumventricular organs (Ganong, 2000).
Blood-Brain Barrier in Pathology and Therapy
When the integrity of the BBB is compromised, the brain becomes exposed to ions, molecules, and chemicals that it normally is not surrounded by. Because the brain is such a complex and sensitive organ, this can have dire consequences. HIV infection may sometimes infect macrophages, a type of immune cell in the body (Persidsky et al., 2006). Consequently, these cells secrete all kinds of signalling molecules that stimulate inflammation. Inflammation normally leads to the dilation of blood vessels so that more immune cells can be transported to the site of infection (Schmid-Schönbein, 2006). In this case, however, the infected macrophages cause alterations in the BBB homeostasis, leading to a less solid barrier. As a result, these HIV-harbouring macrophages, as well as other unwanted molecules, can pass the BBB more easily (Persidsky et al., 2006). This has two major consequences. First, the virus itself will be able to infect cells in the central nervous system. Second, because the virus replicates in the brain and produces viral proteins, inflammation starts to occur in the brain. This is called encephalitis, and it can yield truly horrible symptoms (Strazza et al., 2011). Such symptoms include physical discomfort like fever, or more serious conditions involving seizures. Additionally, encephalitis may lead to personality changes, depression, dementia, and other mental problems (Gultekin, 2000). Besides, changes in the BBB are also manifested in ischemia, Alzheimer’s Disease, brain tumours, and many other neurological diseases (Dong, 2018), which attest to the brain’s sensitivity to anything that can be found in the blood.
On the other hand, even when the BBB functions in the way it is supposed to, it can be a hurdle to deal with. Because transportation across the barrier is so selective, it is very difficult to get any medicine into the brain. Therapeutic molecules can only be of a certain size and weight (Dong, 2017). Many advancements have been made in terms of drug delivery to the brain. Viral vectors, which are manipulated viruses harbouring any gene of interest, have been heavily researched. Nanoparticles have also been synthesised. They are very tiny packages that open up and release drugs when their target site is reached (Dong, 2018). One promising advancement which has not been covered by the media as extensively, is that of the exosome. Exosomes are small vesicles naturally secreted by cells like little garbage bags (Yang et al., 2017). These can be extracted from healthy tissues, and molecules of choice can be inserted into exosomes to effectively work like nanoparticles. The big advantage of exosomes is that they are not immunogenic. In other words, the immune system does not register exosomes as foreign objects, and so will not counteract this therapy (Dong, 2018). In one study performed a few years back, researchers were able to inject RNA into an exosome to inhibit the translation of a protein called vascular endothelial growth factor, or VEGF. This is a key protein in the formation of new vessels, and is often misregulated by tumour cells. These exosomes were successfully used to treat brain tumours in zebrafish. The tumours, in response to this treatment, grew significantly less (Yang et al., 2017). Although many adaptations are required for its use in human patients, research like this marks a very promising start.

Like so much in biology, or science for that matter, the blood-brain barrier is a complex system about which much remains unclear. The interplay of many different cells that together form an impenetrable wall against anything in the blood allows the brain to work in an optimal and well-regulated environment. The disruptions of the BBB’s homeostasis are involved in numerous diseases that affect the central nervous system. This crucial part of the circulatory system, however, is an obstacle to the treatment of diseases in the brain, as drugs in the blood will mostly be filtered out and removed by the BBB. Through recent therapeutic advancements, however, new agents have been identified and are currently being optimised for more efficient drug delivery to the brain.
Bibliography
Cong, X., & Kong, W. (2020). Endothelial tight junctions and their regulatory signaling pathways in vascular homeostasis and disease. Cellular Signalling, 66, 109485. https://doi.org/10.1016/j.cellsig.2019.109485
Daneman, R., & Prat, A. (2015). The blood-brain barrier. Cold Spring Harbor Perspectives in Biology, 7(1), a020412. https://doi.org/10.1101/cshperspect.a020412
Dong, X. (2018). Current strategies for brain drug delivery. Theranostics, 8(6), 1481–1493. https://doi.org/10.7150/thno.21254
Ganong, W. F. (2000). Circumventricular organs: definition and role in the regulation of endocrine and autonomic function. Clinical and Experimental Pharmacology & Physiology, 27(5–6), 422–427. https://doi.org/10.1046/j.1440-1681.2000.03259.x
Gultekin, S. H. (2000). Paraneoplastic limbic encephalitis: neurological symptoms, immunological findings and tumour association in 50 patients. Brain, 123(7), 1481–1494. https://doi.org/10.1093/brain/123.7.1481
Persidsky, Y., Ramirez, S. H., Haorah, J., & Kanmogne, G. D. (2006). Blood–brain barrier: structural components and function under physiologic and pathologic conditions. Journal of Neuroimmune Pharmacology, 1(3), 223–236. https://doi.org/10.1007/s11481-006-9025-3
Sandoo, A., Veldhuijzen van Zanten, J. J. C. S., Metsios, G. S., Carroll, D., & Kitas, G. D. (2010). The endothelium and its role in regulating vascular tone. The Open Cardiovascular Medicine Journal, 4(1), 302–312. https://doi.org/10.2174/1874192401004010302
Schmid-Schönbein, G. W. (2006). Analysis of inflammation. Annual Review of Biomedical Engineering, 8(1), 93–151. https://doi.org/10.1146/annurev.bioeng.8.061505.095708
Strazza, M., Pirrone, V., Wigdahl, B., & Nonnemacher, M. R. (2011). Breaking down the barrier: the effects of HIV-1 on the blood–brain barrier. Brain Research, 1399, 96–115. https://doi.org/10.1016/j.brainres.2011.05.015
Yang, T., Fogarty, B., LaForge, B., Aziz, S., Pham, T., Lai, L., & Bai, S. (2017). Delivery of small interfering RNA to inhibit vascular endothelial growth factor in zebrafish using natural brain endothelial cell-secreted exosome nanovesicles for the treatment of brain cancer. The AAPS Journal, 19(2), 475–486. https://doi.org/10.1208/s12248-016-0015-y
Visual Sources
Cover Image: Wexler, M. (2022). Blood-Brain barrier Signaling Found to Help Regulate Nerve Activity. [Image]. https://parkinsonsnewstoday.com/news/blood-brain-barrier-signaling-regulates-nerve-cells-activity/
Figure 1: Rogers, D., & Lowe, S. (2022). Ultrastructure of Blood Vessels. [Image]. https://teachmeanatomy.info/the-basics/ultrastructure/blood-vessels/
fd
Figure 2: Pathwayz. (n.d.). Homeostasis [Humans]. [Image]. https://www.pathwayz.org/Tree/Plain/HOMEOSTASIS+%5BHUMANS%5D
Figure 3: Dotiwala, A. K., McCausland, C., & Samra, N. S. (2022). Anatomy, Head and Neck, Blood Brain Barrier. In StatPearls (Vol. 3, Issue 8). Cold Spring Harbor Laboratory Press. [Image]. http://www.ncbi.nlm.nih.gov/pubmed/25805644
Figure 4: Luan, X., Sansanaphongpricha, K., Myers, I., Chen, H., Yuan, H., & Sun, D. (2017). Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta Pharmacologica Sinica, 38(6), 754–763. [Image]. https://doi.org/10.1038/aps.2017.12







Comments