Stem Cell Biotechnology Series: Exploring the Potential of Stem Cells
- Victor Cornily

- Jun 25, 2023
- 12 min read
Updated: Sep 11, 2024
Foreword
The field of stem cells is brimming with promise and intrigue. As the understanding of these remarkable foundational components of life continues to evolve, so does the capacity to harness their extraordinary potential. Stem cells have enchanted the minds of scientists, medical professionals, and curious individuals alike, presenting unparalleled opportunities to revolutionise healthcare and regenerative medicine. This series of articles aims to unravel the mysteries of stem cell differentiation, explore the mechanisms governing their self-renewal, and examine the factors that shape their destiny. Within these articles, readers may discover the immense potential of induced pluripotent stem cells in reshaping personalised medicine while uncovering their awe-inspiring regenerative capabilities. Moreover, this series sheds light on the critical challenges and limitations inherently intertwined with this field, from ensuring the safety and efficacy of stem cell therapies through navigating the complex terrain of regulatory frameworks and public perception to debating ethical uncertainties.
This series is divided into eight articles, including:
Stem Cell Biotechnology Series: Exploring the Potential of Stem Cells
Stem Cell Biotechnology Series: Sources and Isolation Methods
Stem Cell Biotechnology Series: Differentiation and Lineage Commitment
Stem Cell Biotechnology Series: Self-Renewal and Maintenance
Stem Cell Biotechnology Series: Induced Pluripotent Stem Cells (iPSCs) and Genetic Engineering
Stem Cell Biotechnology Series: Stem Cells in Regenerative Medicine
Stem Cell Biotechnology Series: Stem Cells in Disease Modelling and Cancer Research
Stem Cell Biotechnology Series: Ethical Considerations and Future Perspectives
Stem Cell Biotechnology Series: Exploring the Potential of Stem Cells
Introduction
In an ever-evolving landscape of healthcare needs, the necessity for tissue replacement and regeneration continues to rise. Age-related conditions, degenerative diseases, tumours, traumatic injuries, and congenital disorders contribute to this growing demand. Amid these challenges, the potential of stem cells shines brightly as a source of optimism and promise. Stem cells, the fundamental building blocks of biological organisation, can drive the development and regeneration of organ and tissue systems. Their unique characteristics, including self-renewal and multi-lineage differentiation, make them invaluable in advanced tissue engineering and cell therapies. Researchers can guide their differentiation towards desired cell types by combining stem cells with synthetic or nature-derived scaffolds carefully tailored in terms of composition, architecture, and physicochemical properties. This outstanding potential is further amplified when coupled with optimised cell culture media and suitable mechanical, electrical, or magnetic stimulation. These advancements are vital to unlocking a future where damaged tissues can be repaired and organ function restored, bringing humankind closer to a new era of regenerative medicine (Bacakova et al., 2018).
History
In 1961, a pivotal moment in scientific history occurred when Dr. James A. Till and Dr. Ernest A. McCulloch, based at the University of Toronto in Canada, first described stem cells. Their ground-breaking research revealed that stem cells derived from mouse bone marrow could differentiate into diverse cell types, earning them the name pluripotent stem cells (PSCs). Building upon this foundation, a significant milestone was achieved in 1996 when Keith Campbell, Ian Wilmut, and their team at the Roslin Institute in Scotland successfully cloned Dolly the sheep. This demonstrated the validity of somatic cell nuclear transfer. Two years later, in 1998, another breakthrough was realised as James Thomson isolated the first human embryonic stem cells (hESCs). The field witnessed further progress in 2006 when induced pluripotent stem cells (iPSCs) were generated by reprogramming adult somatic cells by using just four transcription factors, an impressive reduction from the initial 24 factors. In recognition of their transformative discovery that mature cells could be reprogrammed into a pluripotent state, Shinya Yamanaka and John Gurdon were awarded the Nobel Prize for Physiology or Medicine in 2012. Henceforth, researchers have identified innate adult stem cells within various organs (Liu et al., 2020). Figure 1 depicts the progression of these historical developments, with fundamental research represented by red shading, preclinical work by yellow shading, and clinical trials by green shading.


Figure 1. The timeline of major scientific advances during the history of stem cell research. (Liu, 2020)
Stem cell classification, terminology, biology
To understand the potential of stem cells, it is essential to consider their capacity to differentiate into specific cell types within the body. That ability is defined by particular terminology used in the field. Totipotency refers to the ability to differentiate into all types of cells, including placental cells. Pluripotency allows for the creation of any tissue in the body except for the placenta. Multipotent stem cells exhibit a more limited range of differentiation as they can specialise into specific cell types within distinct lineages. For instance, haematopoietic stem cells can develop into various types of blood cells. Once they differentiate, haematopoietic stem cells become oligopotent cells, which means their differentiation potential is restricted to cells within their own lineage. Unipotent stem cells possess the narrowest range of differentiation capabilities meaning they can only generate exactly one cell type. Nevertheless, they possess a unique characteristic of undergoing repeated division. This property makes them highly promising for therapeutic applications in regenerative medicine (Zakrzewski et al., 2019).

Figure 2. From ovulation to implantation. (Homer, n.d.)
Following the fusion of sperm and ovum during fertilisation, a blastocyst is formed. Within the inner wall of the blastocyst, there exist short-lived stem cells known as embryonic stem cells (ESCs). The blastocyst comprises two distinct cell types: the inner cell mass, responsible for the development of the foetus, specifically the epiblasts, and the trophectoderm. Human embryonic stem cells are derived from the ICM. During embryogenesis, cells organise into aggregations called germ layers: endoderm, mesoderm, and ectoderm. Each germ layer ultimately gives rise to specialised cells and tissues in the foetus and, subsequently, the adult organism (Zakrzewski et al., 2019).

Figure 3. The structure of a blastocyst. (Homer, n.d.)
Once hESCs differentiate into one of these germ layers, they transition into multipotent stem cells, which possess a limited potential and can only generate cells within their respective germ layer. This developmental process occurs relatively rapidly in humans. Following this stage, pluripotent stem cells are dispersed throughout the organism as undifferentiated cells. They possess two fundamental abilities: proliferation, allowing them to generate the next generation of stem cells, and differentiation, enabling them to develop into specialised cells under specific physiological conditions. Signals influencing the process of stem cell specialisation can be categorised as external, such as physical interactions between cells or chemical signals from surrounding tissues, and internal, which encompass signals controlled by genes within the DNA (Zakrzewski et al., 2019).
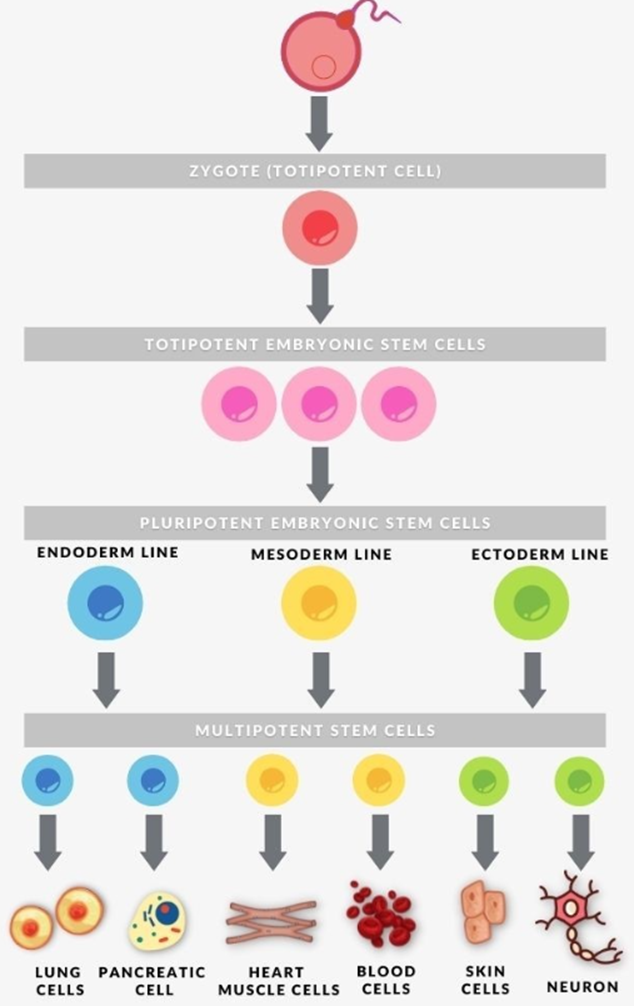
Figure 4. Cells' potency. (Gonzaga, n.d.)
Five main categories of stem cells exist —embryonic stem cells (ESCs), very small embryonic-like stem cells (VSELs), nuclear transfer stem cells (NTSCs), reprogrammed stem cells (RSCs), and adult stem cells (ASCs). Notably, only NTSCs have demonstrated the ability to generate a complete organism, as evidenced by the successful growth of monkeys from NTSCs in China in 2018. On the other hand, ESCs, iPSCs, and ASCs have been primarily used for tissue and organ generation. ESCs and iPSCs, in particular, have shown tremendous potential in various applications, including regenerative and transplant medicine, disease modelling, drug discovery screening, and human developmental biology. The field constantly evolves —from the initial descriptions of stem cells to their expanding clinical applications today (Liu et al., 2020).
Table 1. Five Basic Categories of Stem Cells. (Liu, 2020)


The distinction between a stem cell and a differentiated cell is evident in their DNA composition. Stem cells have loosely arranged DNA with active genes, while differentiated cells shut down unnecessary genes and retain active genes relevant to their specialised function. This process of pluripotency can be reversed by manipulating gene sequences, as demonstrated by the discovery of key transcription factors like Oct3/4, Sox2, and Nanog, which play a crucial role in maintaining pluripotency and generating induced pluripotent stem cells (Takahashi & Yamanaka, 2006). The extraordinary properties and full characterisation of iPSCs will be reviewed in a separate article in this series.
Clinical Application
Improper cell differentiation or division can lead to severe medical conditions such as congenital disabilities and cancer. However, the emergence of stem cell therapies offers a ray of hope for addressing these challenges. From spinal cord injuries to heart failure, retinal and macular degeneration, tendon ruptures, and type 1 diabetes, stem cell treatments hold promise in tackling a wide range of ailments (Zakrzewski et al., 2019).
Stem cells offer distinct advantages in regenerative medicine. They can be directly applied to damaged areas for cell therapy or incorporated into suitable scaffolds for tissue engineering. Compared to differentiated cells, stem cells are more readily obtainable on a larger scale, possess a higher capacity for proliferation, exhibit greater endurance during passages, experience senescence at a later stage, can differentiate into a wide range of desired cell types, and promote scaffold vascularisation. These unique characteristics make stem cells a valuable resource for advancing regenerative medicine (Zakrzewski et al., 2019).

Figure 5. Stem cell experiments on animals. (Zakrzewski et al., 2019)
For stem cells to be therapeutically effective, they need to be transformed into specific cell types as required, as the entire process of regenerative medicine would otherwise be futile. The differentiation of ESCs is particularly crucial, as undifferentiated ESCs can lead to the formation of teratomas in the body. Successful regenerative medicine means comprehending and utilising signalling pathways that drive differentiation. Directed differentiation involves mimicking the signals cells receive during their natural developmental stages. The extracellular microenvironment also plays a significant role in governing cell behaviour. By manipulating the culture conditions, it becomes possible to guide specific differentiation pathways and generate cell cultures enriched with desired precursor cells in laboratory settings. Unfortunately, replicating the same effect in the in vivo environment presents challenges. Therefore, it is crucial to develop culture conditions that facilitate homogeneous and enhanced differentiation of ESCs into functional and desired tissues (Cohen & Melton, 2011).
Figure 6 presents two primary approaches for producing patient-specific cells of a desired type. In regenerative medicine, pluripotent cells can be obtained from either the patient, that is, iPSCs, or from non-patient sources such as ESCs or iPSCs. These pluripotent cells can then undergo directed differentiation in a controlled laboratory environment to attain the desired cell state (depicted on the right side of the figure). Alternatively, primary cells derived directly from the patient can be reprogrammed to generate the desired cell type (depicted on the left side of the figure). Cells of the desired type obtained through either of these methods can be further studied in vitro (shown at the bottom of the figure) or utilised for transplantation into patients (shown at the top).

Figure 6. The central strategies of regenerative medicine. (Cohen & Melton, 2011)
Hematopoietic stem cells hold great importance as they have been extensively studied for over 50 years and serve as a well-characterised model system for tissue-specific stem cells. They offer valuable insights into the study of tissue-specific stem cells and hold potential in regenerative medicine. Currently, the most widely practised stem cell therapy involves transplanting multipotent hematopoietic stem cells (Zakrzewski et al., 2019).
Stem cells offer the potential for various applications, including their use in new drug testing. By utilising specific differentiated cells derived from pluripotent cells, experiments can be conducted on living tissue safely. This approach allows for adjustments to the drug formulas until they reach optimal effectiveness, ensuring that the drugs entering the market do not harm any live testers. To properly compare the effects of different drugs, it is crucial to maintain similar conditions. Researchers aim to gain complete control over the differentiation process to generate pure populations of differentiated cells (Zakrzewski et al., 2019).
In regenerative medicine, stem cells provide an alternative for addressing problems related to tendons, osteoarthritis, and osteonecrosis of the femoral hip (Filomeno et al., 2012). Notably, Hanataka et al. demonstrated the effectiveness of using Oct4, Sox2, Klf4, and C-myc genes to induce regeneration in the pancreas and skeletal muscle cells with limited regenerative capacity (Hatanaka et al., 2016).
Stem cells can be induced to differentiate into specific cell types required for repairing damaged or destroyed tissues. This approach presents a promising solution to the shortage of transplantable tissues and organs. Various conditions, such as macular degeneration, strokes, osteoarthritis, neurodegenerative diseases, and diabetes, can potentially benefit from stem cell therapy. For example, stem cells can be differentiated into healthy heart muscle cells for transplantation in patients with heart disease (Yamakawa & Ieda, 2021). Similarly, in the case of type 1 diabetes, stem cells can be prompted to differentiate into insulin-producing cells as an alternative to transplantation therapy (Shahjalal et al., 2018).
In 2011, Hayashi et al. demonstrated the possibility of generating sperm from iPSCs in mice, resulting in the birth of healthy and fertile pups (Hayashi et al., 2011). Additionally, Zhang et al. provided evidence that transplantation of human amniotic epithelial cells effectively improved ovarian function in mice, offering the potential for managing ovarian issues in female cancer survivors (Zhang et al., 2015).
Moreover, stem cell therapy can delay the progression of incurable neurodegenerative diseases and address their underlying causes. Conditions such as Parkinson's disease, multiple sclerosis, Alzheimer's disease, amyotrophic lateral sclerosis, and Huntington's disease can all benefit from this approach. The discovery of neural stem cells has challenged the notion that the adult central nervous system lacks neurogenesis, as neurogenesis has been observed throughout life (Dantuma et al., 2010).
There is a possibility that stem cells surpass synthetic materials in widespread usage. Teeth, in particular, serve as a natural and non-invasive source of stem cells. Dental stem cell groups, including dental pulp stem cells, periodontal ligament stem cells, stem cells from apical papilla, and dental follicle stem cells, can be isolated and studied for various applications (Zakrzewski et al., 2019).

Figure 7. Localisation of stem cells in dental tissues. (Zakrzewski et al., 2019)
Conclusions
Given stem cells’ potential applications and exceptional characteristics, it is no surprise that this field is highly regarded and esteemed. They have been described with such praising descriptions as ‘the holy grail’ (Berebichez-Fridman et al., 2017) or ‘turning straw into gold’ (Cohen & Melton, 2011). It is easy to succumb to the enthusiasm, and this optimism is well-founded. After years of experimentation, stem cell therapy is emerging as a tremendous transformative force in medicine. With each new experiment, the capabilities of stem cells continue to expand, even though numerous challenges still lie ahead. Nevertheless, the impact of stem cells in the realms of regenerative medicine and transplantology is immense. Whilst it would be premature to make absolute claims about stem cells being the miraculous remedy of the future, they will undoubtedly maintain a strong presence in prospective therapies.
Bibliographic references
Bacakova, L., Zarubova, J., Travnickova, M., Musilkova, J., Pajorova, J., Slepicka, P., Kasalkova, N. S., Svorcik, V., Kolska, Z., Motarjemi, H., & Molitor, M. (2018). Stem cells: their source, potency and use in regenerative therapies with focus on adipose-derived stem cells – a review. Biotechnology Advances, 36(4), 1111–1126. https://doi.org/10.1016/j.biotechadv.2018.03.011
Berebichez-Fridman, R., Gómez-García, R., Granados-Montiel, J., Berebichez-Fastlicht, E., Olivos-Meza, A., Granados, J., Velasquillo, C., & Ibarra, C. (2017). The Holy Grail of Orthopedic Surgery: Mesenchymal Stem Cells - Their Current Uses and Potential Applications. Stem Cells International, 2017. https://doi.org/10.1155/2017/2638305
Cohen, D. E., & Melton, D. (2011). Turning straw into gold: Directing cell fate for regenerative medicine. Nature Reviews Genetics, 12(4), 243–252. https://doi.org/10.1038/nrg2938
Dantuma, E., Merchant, S., & Sugaya, K. (2010). Stem cells for the treatment of neurodegenerative diseases. Stem Cell Research and Therapy, 1(5), 1–7. https://doi.org/10.1186/scrt37
Filomeno, P., Dayan, V., & Touriño, C. (2012). Stem cell research and clinical development in tendon repair. Muscles, Ligaments and Tendons Journal, 2(3), 204–211.
Hatanaka, F., Hishida, T., Li, M., Lam, D., Kurita, M., Beyret, E., Araoka, T., Vazquez-ferrer, E., Donoso, D., Luis, J., Xu, J., Esteban, C. R., Nuñez, G., Nuñez, E., Campistol, J. M., Guillen, I., Guillen, P., Belmonte, I. J. C., Ocampo, A., … Martinez, R. (2016). In Vivo Amelioration of Age-Associated Hallmarks by Partial Reprogramming. Cell, 167(7), 1719–1733. https://doi.org/10.1016/j.cell.2016.11.052.In
Hayashi, K., Ohta, H., Kurimoto, K., Aramaki, S., & Saitou, M. (2011). Reconstitution of the mouse germ cell specification pathway in culture by pluripotent stem cells. Cell, 146(4), 519–532. https://doi.org/10.1016/j.cell.2011.06.052
Liu, G., David, B. T., Trawczynski, M., & Fessler, R. G. (2020). Advances in Pluripotent Stem Cells: History, Mechanisms, Technologies, and Applications. Stem Cell Reviews and Reports, 16(1), 3–32. https://doi.org/10.1007/s12015-019-09935-x
Shahjalal, H. M., Abdal Dayem, A., Lim, K. M., Jeon, T. Il, & Cho, S. G. (2018). Generation of pancreatic β cells for treatment of diabetes: Advances and challenges. Stem Cell Research and Therapy, 9(1). https://doi.org/10.1186/s13287-018-1099-3
Takahashi, K., & Yamanaka, S. (2006). Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell, 126(4), 663–676. https://doi.org/10.1016/j.cell.2006.07.024
Yamakawa, H., & Ieda, M. (2021). Cardiac regeneration by direct reprogramming in this decade and beyond. Inflammation and Regeneration, 41(1). https://doi.org/10.1186/s41232-021-00168-5
Zakrzewski, W., Dobrzyński, M., Szymonowicz, M., & Rybak, Z. (2019). Fuel Cells: Past, Present and Future. Stem Cell Research and Therapy, 128(5), 329–332. https://doi.org/10.1541/ieejfms.128.329
Zhang, Q., Xu, M., Yao, X., Li, T., Wang, Q., & Lai, D. (2015). Human amniotic epithelial cells inhibit granulosa cell apoptosis induced by chemotherapy and restore the fertility. Stem Cell Research and Therapy, 6(1), 1–10. https://doi.org/10.1186/s13287-015-0148-4
Visual references
Cover Image. 3D abstract illustration of scientific collage. (n.d.) [Illustration]. Pixabay. https://pixabay.com/illustrations/ai-generated-cell-blood-virus-7813439/
Figure 1. The timeline of major scientific advances during the history of stem cell research. G., David, B. T., Trawczynski, M., & Fessler, R. G. (2020). [Illustration]. Advances in Pluripotent Stem Cells: History, Mechanisms, Technologies, and Applications. Stem Cell Reviews and Reports, 16(1), 3–32. https://doi.org/10.1007/s12015-019-09935-x
Figure 2. Homer, H. (n.d.) From ovulation to implantation. [Illustration]. Doctor Hayden Homer. https://www.drhaydenhomer.com.au/hrf_faq/what-happens-to-the-embryo-after-fertilisation/
Figure 3. Homer, H. (n.d.) The structure of a blastocyst. [Illustration]. Doctor Hayden Homer. https://www.drhaydenhomer.com.au/hrf_faq/what-happens-to-the-embryo-after-fertilisation/
Figure 4. Gonzaga, M. (n.d.) Cells' potency. [Illustration]. Biology Online. https://www.biologyonline.com/dictionary/totipotent
Figure 5. Stem cell experiments on animals. Zakrzewski, W., Dobrzyński, M., Szymonowicz, M., & Rybak, Z. (2019). [Illustration]. Fuel Cells: Past, Present and Future. Stem Cell Research and Therapy, 128(5), 329–332. https://doi.org/10.1541/ieejfms.128.329
Figure 6. The central strategies of regenerative medicine. Cohen, D. E., & Melton, D. (2011). [Illustration]. Turning straw into gold: Directing cell fate for regenerative medicine. Nature Reviews Genetics, 12(4), 243–252. https://doi.org/10.1038/nrg2938
Figure 7. Localisation of stem cells in dental tissues. Zakrzewski, W., Dobrzyński, M., Szymonowicz, M., & Rybak, Z. (2019). [Illustration]. Fuel Cells: Past, Present and Future. Stem Cell Research and Therapy, 128(5), 329–332. https://doi.org/10.1541/ieejfms.128.329




The decision by OpenAI to utilize Kenyan workers for less than $2 per hour is deeply concerning and unethical. This exploitation of labor not only perpetuates a cycle of poverty but also undermines the value of the work being done, look more at https://247newsaroundtheworld.com/news/openai-used-kenyan-workers-on-less-than-2-per-hour-exclusive/ . It is disappointing to see a company with such potential resort to such exploitative practices. As consumers, we must hold companies accountable for their actions and demand fair treatment of all workers involved in their operations.