Principles of Neuroscience: Neurotransmitters - How to Get the Message Across
- Maria McGovern

- Sep 17, 2023
- 19 min read
Updated: Sep 20, 2024
Neurotransmitters are the endogenously produced chemical messengers that carry signals between neurons. As brain function is facilitated by neurotransmitters, their receptors and proteins involved in the synthesis or inactivation of neurotransmitters are key targets for manipulating brain function. This is reflected in pharmacological therapies used to treat neurological and psychiatric disorders including pain regulation and antidepressants (Hyman, 2005). The body relies on neuronal signalling to carry out nearly every function. Electrical signals arriving in the axon terminal stimulate the release of neurotransmitters into the synaptic cleft, the space between two neurons. These neurotransmitters transmit this message as a chemical signal across the synapse to bind to receptors on the next neuron. The signal is then converted back into an electrical signal to be carried to the next cell and on and on until the signal reaches its destination (Li & Cho, 2011).
Neurotransmitters
Neurotransmitter transmissions are a vital part of shaping the neuronal network structure. Both neurotransmitters and neuromodulators (molecules that work with neurotransmitters to enhance signals) play an important role in shaping and wiring the nervous system throughout neural development. Neurotransmitters and neuromodulators affect synaptic formation, synaptic maturation and synaptic pruning through electrical activity and excitability regulation. New synapses are formed between the axon of a presynaptic neuron and the dendrite of a postsynaptic neuron. When a new synapse is formed, there is an increase in both neurotransmitters and neuromodulators in this region. In the early stages of life, when most synapses are formed, there is a rapid increase in some types of neurotransmitters, such as glutamate, acetylcholine, and neuropeptides, at these synapses. For example, at birth, there is an influx of noradrenaline which may be responsible for the initial fast bonding of a newborn child to its mother. The amount of these neurotransmitters reduces and becomes more level to other neurotransmitters as more synapses are established (Herlenius & Lagercrantz, 2004).

There are hundreds of molecules that have been identified and classified as neurotransmitters so far. Neurotransmitters can exert excitatory or inhibitory inputs to neurons, which affects the nervous system’s generation and degeneration of new synapses. Excitatory neurotransmitters excite the neuron and enable electrical signals to be transmitted to the next neuron. Inhibitory neurotransmitters, on the other hand, stop the propagation of the signal and prevent it from being passed on any further. There are also modulatory neurotransmitters, which can adjust the signals of other neurotransmitters and chemical messengers and can affect a large amount of cells at the same time. The effect of signals depends on the type of neurotransmitter that transmits them. Excitatory neurotransmitters include glutamate and norepinephrine, while GABA (gamma-aminobutyric acid) and serotonin are examples of inhibitory neurotransmitters. Dopamine and acetylcholine can have both inhibitory and excitatory effects (Hyman, 2005). Mattson and Kater (1989) showed the impact of neurotransmitters on dendritic growth and cell survival. In this study, neurons exposed to glutamate (excitatory) and GABA (inhibitory) were monitored. Glutamate showed graded changes in dendrites. With low doses of glutamate, selective inhibition in dendritic outgrowth and dendritic pruning occurred, while high doses of glutamate resulted in cell death. GABA, on the other hand, did not affect dendrite outgrowth at low doses and higher doses only moderately reduced dendritic outgrowth. However, GABA did significantly reduce the dendritic impairment and cell death that glutamate caused. This study suggests that neurotransmitter activity can dramatically impact synaptic formation and degeneration.
There are six major neurotransmitters: acetylcholine, dopamine, norepinephrine, serotonin, gamma-aminobutyric acid (GABA) and glutamate. (Hyman, 2005). Based on their chemical makeup, neurotransmitters can be amino acids (such as glutamate, GABA and glycine), biogenic amines (for example dopamine, norepinephrine, epinephrine and serotonin) or neuropeptides (like substance P, neuropeptide Y, and opioids, among many others). Amino acid and biogenic amines are ‘classic’ neurotransmitters, while neuropeptides are messenger molecules that provide additional support to classic neurotransmitters (Li & Cho, 2011). Neuropeptides co-exist with other neurotransmitters, acting as both transmitters and trophic factors (aid cellular processes). They are particularly important when the nervous system is under pressure, such as when injury, pain or stress occur (Hökfelt et al., 2018).

There are certain criteria a chemical molecule must meet to be classified as a neurotransmitter: it must be found on presynaptic neurons, it must be released in a calcium-dependent process from the presynaptic terminals when the neuron is depolarised, there must be mechanisms for its inactivation, it must be able to mimic synaptic responses exogenously and agonists or antagonists must be able to enhance or block, respectively, the neurotransmitters response (Avoli & Krnjević, 2016; Robinson & Coyle, 1987). Some neurotransmitters—like serotonin, norepinephrine, and dopamine—are strongly associated with mood. The levels of these neurotransmitters are directly linked to happiness or depression, to the point that they are excellent targets for antidepressants. Many antidepressants target the reuptake of neurotransmitters from the synaptic cleft which will be discussed later in this article.
Signal Transmission
Neuronal cells have been specialised to work with neurotransmitters to receive, process, and transmit information. Within the neuron, this information is electrical, and it is converted into chemical information when this signal is released into the synapses via neurotransmitters. Most synapses are chemical, but there are also electrical synapses. Electrical synapses use ion flows between cells to transmit simple signals through gap junctions. Chemical synapses, on the other hand, can exert excitatory, inhibitory, and other complex biochemical signals between cells (Hyman, 2005). Typically, neurotransmitters are released from a presynaptic cell into the synaptic cleft and bind to receptors on the postsynaptic cell. An action potential (AP) arriving in the presynaptic axon terminal causes the vesicle in which the neurotransmitters are stored to fuse with the cell membrane and dispense those neurotransmitters into the synaptic cleft. The neurotransmitters then bind to receptors, usually on a dendrite of the postsynaptic cell. This chemical information is converted into electrical information again, by activating ion channels. Receptors themselves can carry the function of ion channels, as in the case of GABA receptors that open upon GABA binding. Many molecular structures have been shown to act as neurotransmitter receptors, but the most common are ligand-gated channels and G-protein-coupled receptors (Hyman, 2005).

Neurotransmitter signalling can be impaired due to disease or disorders that affect the amount of a particular neurotransmitter being synthesized. This occurs in neurodegenerative diseases, such as Parkinson’s disease or Alzheimer’s disease, where the neurons carrying certain neurotransmitters are degraded, resulting in a reduction in the availability of that neurotransmitter. The association of Parkinson’s with reduced dopamine, and Alzheimer’s with reduced acetylcholine is discussed later on in the article. Other causes of neurotransmitter signalling impairment include dysfunction of neurotransmitter receptors, which prevents the signal transmitted from reaching the next cell. Overactive reuptake can prevent neurotransmitters from transmitting the information before they are transported back to the cell. Inflammation causing damage to the synaptic cleft can also impair this signalling, as seen in myasthenia gravis, a condition where muscles are weakened (Hyman, 2005).
Acetylcholine
The first neurotransmitter to be detected was acetylcholine, in 1926, when Otto Loewi discovered that this molecule carried chemical signals from the vagus nerve (a cranial nerve) to the heart that slowed down the heart rate (Hyman, 2005). Later, Eccles and colleagues (1954) showed that acetylcholine was released from motor neurons to bind to cells of the spinal cord. Acetylcholine is vital for many functions in the CNS, particularly in learning and memory. During reward-based learning, acetylcholinergic activity in the striatum is increased. This neurotransmitter can have excitatory and inhibitory effects (Deiana et al., 2011).

As the first identified neurotransmitter, acetylcholine has been well investigated and more is known about its receptors than any other neurotransmitter receptors. Nicotinic and muscarinic receptors are examples of acetylcholine receptors. However, they are very pharmacologically different. The nicotinic receptor is an ion channel composed of pentameric subunits, while the muscarinic receptor is a G-protein-coupled receptor (Brown, 2006). Although acetylcholine can work through both muscarinic and nicotinic systems for spatial learning, there is no clear difference in the effect of the two different systems on the learning outcome (Deiana et al., 2011). Working memory and short-term memory both engage the nucleus basalis—prefrontal cortex—acetylcholine system. In this system, the axons of cholinergic neurons from the nucleus basalis magnocellularis (NBM) are projected throughout the cerebral cortex, olfactory bulbs, and the amygdala (Wenk, 1997).
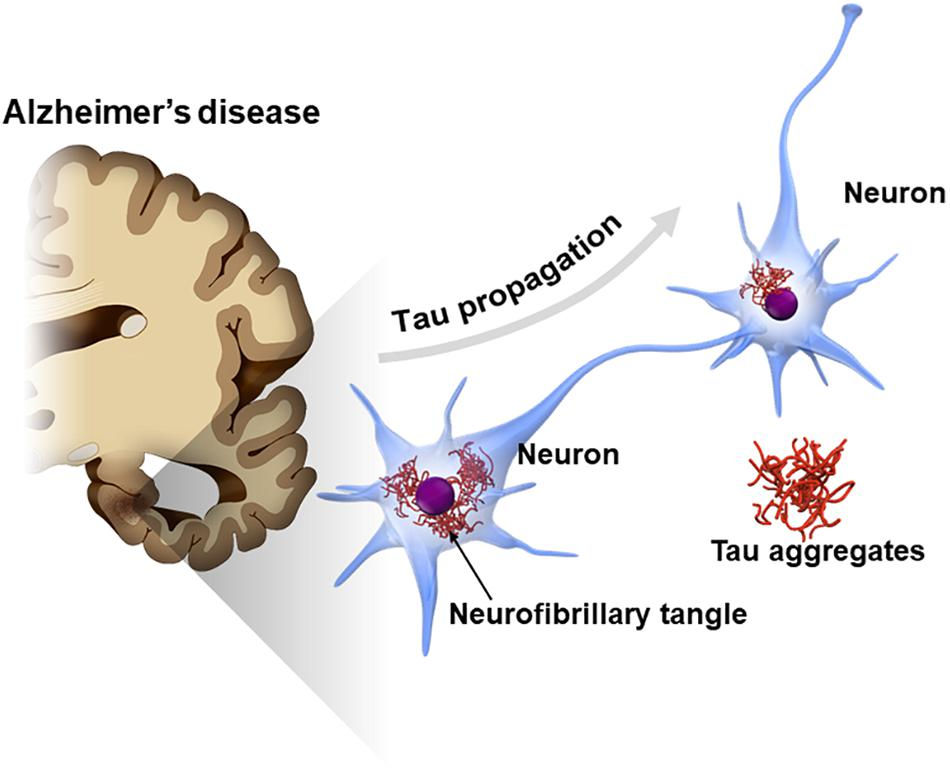
Studies that lesioned the NBM resulted in significantly reduced ability to focus on a task. This revealed that acetylcholine signalling in this region controls how attention shifts by modulating sensory inputs from the midbrain and limbic system (includes the amygdala, hippocampus, and hypothalamus and mediates emotion and cognition) to determine where attention should be focused. Further electrophysiological evidence has shown acetylcholine projections from the NBM also enable the control and maintenance of sleep states (Wenk, 1997). The connection of the NBM to the limbic system may, however, leave it vulnerable to abnormal build-up of Tau protein and neurofibrillary degeneration seen in Alzheimer's disease. The loss of these cholinergic neurons further exacerbates this disease (Mesulam, 2013).

Acetylcholine may be involved in the aetiology of certain psychiatric disorders, such as schizophrenia. Cholinesterase enzymes stop cholinergic neuron signalling by breaking acetylcholine down into choline and acetic acid. However, cholinesterase inhibitors, like physostigmine, can be used to decrease manic symptoms by increasing levels of acetylcholine. This drug also causes severe depression and slows motor response. Physostigmine also causes lethargy, slowed thoughts, withdrawal, apathy, decreased energy, and feeling drained. Patients were also observed to become less friendly and talkative while taking this drug. Acetylcholine is therefore shown to impair these functions at increased levels (Janowsky et al., 1974).
Acetylcholine-driven learning can be impaired by acetylcholine receptor antagonists, while agonists can improve memory defects or even, in the right conditions, be used as general cognitive enhancers to improve attention, learning and memory. Due to acetylcholine’s role in memory, the cholinergic system is key to current treatments for diseases affecting memory and cognition, like Alzheimer’s. These treatments, such as cholinesterase inhibitors, increase available acetylcholine and preserve memory. Stimulation of nicotinic receptors results in increased cognition. As the name suggests, nicotinic receptors are also stimulated by nicotine, and the stimulating effects are experienced by tobacco users. The effects seen in smokers have prompted investigations into nicotinic receptors as targets in the treatment of schizophrenia, Alzheimer’s and attention deficit hyperactivity disorder. The stimulation of these receptors with nicotinic agonists has shown significant improvements in attention, working memory and executive processes. Mutations in genes encoding nicotinic receptor subunits are associated with cognitive deficits, further reiterating the role of acetylcholine and nicotinic receptors in learning and cognition (Wallace & Bertrand, 2013).
Dopamine
Dopamine, like acetylcholine, can act as both inhibitory and excitatory neurotransmitter. Dopamine receptors are G-protein-coupled receptors (GPCRs), named D1-D5, that can trigger two opposing signalling cascades, which can increase or decrease glutamate signal strength, depending on the G-protein activated (Rizzi & Tan, 2017). Dopamine transmits signals for learning, motivation and control of body movement. When used in the motivation/reward system, dopamine is produced in the ventral tegmental area (VTA, nerve cell area in the brain) and is then released from the VTA to the prefrontal cortex and nucleus accumbens. When used for motor and movement signalling, dopamine is produced in the substantia nigra and released into the striatum (Latif et al., 2021).
How the body distinguishes whether dopamine signalling is for motivation, movement or learning is debated by researchers. One school of thought claims that slow (‘tonic’) dopamine transmissions are involved with motivation, while fast (‘phasic’) transmissions are a part of learning. Other studies suggest the effect of dopamine signalling has more to do with the receptors that bind this neurotransmitter (Berke, 2018).

Disruption of dopamine signalling pathways has shown immediate and severe movement impairment. Parkinson’s Disease (PD) is a progressive neurodegenerative disease associated with a loss of dopamine neurotransmitters. PD is characterised by akinesia (movement impairment), bradykinesia (slowed movement), resting tremors and rigidity (Bloem et al., 2021). Although there is of yet no cure for this debilitating disease, the most common treatment for PD symptoms is dopamine replacement drugs, such as Levodopa. However, the first stages of PD involve the loss of non-dopaminergic neurons, suggesting that although replacing dopamine can reduce PD symptoms and improve movement ability in patients, better treatments for PD may be found in the early stages of the disease, before dopamine has depleted too far (Ahlskog, 2007).
Glutamate
Glutamate is the main excitatory neurotransmitter in the brain (Prescot et al., 2009). Unlike most other neurotransmitters, glutamate is found in a wide range of tissues in the body, which made the process of classifying glutamate as a neurotransmitter long and difficult. Sufficient evidence has since been established for glutamate to meet the criteria (Robinson & Coyle, 1987). There are three types of ionotropic glutamate receptors: N-methyl D-aspartate (NMDA), kainate, and a-amino-3-hydroxy-5-methyl-4-isoxazolepropionate (AMPA) receptors, each named after their agonists. NMDA receptors are the ones researchers have got to understand the best; they are macromolecular complexes containing an agonist recognition site, modulator sites and a channel. These receptors are involved in long-term potentiation more than neuronal signalling. The other glutamate receptors, kainate and AMPA, play bigger roles in synaptic transmission. Kainate receptors potentiate the excitatory effects of glutamate by triggering the release of glutamate and aspartate from the presynaptic cell (Robinson & Coyle, 1987). Furthermore, these receptors produce sustained excitatory CNS responses, while neuronal responses to AMPA desensitise quickly and affect sensory ganglion neurons. Disruptions of these glutamate receptors can cause neurological disorders like stroke, ischemia (reduced blood flow) and neural degeneration (Bettler & Mulle, 1995).

Changes in the production of glutamate can have pathophysiological effects and cause conditions such as migraines. Increased concentrations of excitatory amino acid neurotransmitters in neuronal synapses can cause overactivity of the NMDA Glu receptor subtype. Increased activity of this receptor subtype has been shown to amplify pain transmission of migraines. However, this receptor can also be key to reducing this pain. Inhibiting the NMDA receptor with low-affinity antagonists, such as memantine, can reduce the frequency and intensity of migraines and tension-type headaches (Prescot et al., 2009).
Norepinephrine
Norepinephrine plays a key role in determining executive function and regulating cognition and motivation and is associated with mood and happiness through its effects on the limbic system. This neurotransmitter regulates the excitability of neurons in the basolateral amygdala through its involvement in GABA release (Dfarhud et al., 2014). Noradrenergic pathways begin in cell bodies in the locus coeruleus (LC), and project throughout the cerebrum and to the spinal cord. These neurons also project to the limbic system. In patients suffering from depression, norepinephrine signalling in the limbic system is disturbed and affects appetite, response to pain, sexual satisfaction, and can increase aggression (Moret & Briley, 2011). Some antidepressants, such as the selective norepinephrine reuptake inhibitor (reboxetine) are used to improve the mood status of patients (Dfarhud et al., 2014). Under normal conditions, norepinephrine acts only as a sympathetic neurotransmitter. The normal plasma concentration of norepinephrine is 70 to 1700 pg/mL. However, during times of stress, such as during exercise or illness, concentrations can reach 1,800 pg/ml and norepinephrine acts as a hormone (Silverberg et al., 1978).

Although PD is most associated with dopaminergic neuron loss, PD causes a massive loss of cells in the LC, which contains most of the noradrenergic neurons of the brain. Norepinephrine has a neuroprotective role in preventing against loss of other neurons. LC degeneration and loss of norepinephrine neurons in this area make dopamine neurons vulnerable to degradation and damage. Animal models where both dopamine and norepinephrine pathways were lesioned showed more pronounced PD symptoms than by lesioning dopamine alone (Rommelfanger & Weinshenker, 2007). This suggests that dopamine and norepinephrine both contribute to the motor and behavioural defects of PD. The behavioural symptoms seen in PD, such as depression, disrupted sleep and mood swings can be linked to LC degradation. This suggests that the loss of norepinephrinergic neurons in the LC is a major turning point in PD progression (Rommelfanger & Weinshenker, 2007). Depression in this case can be treated using antidepressants targeting the selective norepinephrine transporter (NET), such as the NET blocker reboxetine. Therefore, there is a potential for PD treatment in protecting and restoring norepinephrine to slow the progression of PD. By only focusing on dopamine loss, potential treatment targets for PD could be missed (Rommelfanger & Weinshenker, 2007).
GABA
GABA is the main inhibitory neurotransmitter in the brain. Released GABA can bind and activate GABA receptors on pre or postsynaptic neurons. There are three types of GABA receptors: A, B, and C. GABA-A activates ionotropic anionic channels while GABA-B receptors act through secondary messengers and activate metabotropic channels. In addition, GABA-B receptors on presynaptic cells mediate neurotransmitter release. GABA-C receptors activate ionotropic channels but are usually only found in the retina, although some inhibitory functions of GABA-C receptors have been demonstrated in the adult hippocampus (Avoli & Krnjević, 2016). GABA is also the main neurotransmitter involved in the circadian timing system (sets physical, mental, and behavioural changes in a 24-hour cycle) (Moore & Speh, 1993). There are differences in the subunit compositions of GABA-A receptors, depending on the regions of the brain they are located in. These structural variations imply that there are differences in the functional and pharmacological characteristics of these receptors (Avoli & Krnjević, 2016).

The role of GABA as an inhibitory neurotransmitter makes GABA receptors ideal targets for many drugs.(Avoli & Krnjević, 2016). Some of the most common drug types that act on GABA receptors are benzodiazepines, barbiturates and general anaesthetics. These drugs reduce neuronal activity in the brain by positively modulating GABA-A receptors, resulting in sedating effects. For instance, in the 1970s, it was discovered that benzodiazepines enhanced GABAergic inhibition in the central nervous system (CNS). Benzodiazepines are GABA-A receptor ligands that can be used to treat epilepsy, anxiety and insomnia (Kim et al., 2020; Whiting, 2003). This showed that benzodiazepine ligands are positive allosteric modulators that can be used to modulate ionotropic GABAergic function. Since then, other substances, including barbiturates, steroid metabolites and ethanol, have been shown to modulate GABA-A receptor function (Avoli & Krnjević, 2016; Whiting, 2003).
Serotonin
Serotonin is a biogenic amine with a vast array of roles in the body and is found throughout the animal kingdom. This molecule can act as a hormone, a neurotransmitter, and a mitogen (a molecule that induces cell division) with roles in modulating gastrointestinal motility, peripheral vascular tone, cerebral vascular tone, and platelet function. It is also therefore implicated in emesis (vomiting), migraine, and pulmonary and systemic hypertension. Serotonin is, however, most known for its roles in the pathophysiology of mood disorders (Mohammadzadeh et al., 2008).

Serotonin synthesis can occur in the CNS or in the periphery. In the CNS serotonin is synthesized and stored in presynaptic neurons, specifically in serotonergic neurons, pineal gland, and catecholaminergic neurons. In the periphery serotonin synthesis occurs in enterochromaffin cells. About 95% of serotonin is stored in the periphery. There is very little serotonin circulating in the plasma as platelets readily take up serotonin, while the levels of serotonin in the body tissues depend on the rate of synthesis and the rate of metabolism (Mohammadzadeh et al., 2008). Platelets store large amounts of serotonin but have only a limited ability to produce this neurotransmitter (Mohammadzadeh et al., 2008).
Like other neurotransmitters, serotonin is released into the synaptic cleft when the neuron it is stored in depolarises. Then serotonin can bind to postsynaptic serotonin receptors or presynaptic serotonin autoreceptors. When serotonin binds to autoreceptors, it prompts negative feedback which prevents any more serotonin from being released. The serotonin transporter (SERT), a highly selective transporter can then remove serotonin from the synaptic cleft and transport it back to the presynaptic neuron where it is recycled and stored in the presynaptic vesicles until the cell is depolarised again (Mohammadzadeh et al., 2008).
The association of serotonin with mood has established serotonin as a mediator for satisfaction, happiness and optimism. High levels of serotonin are in those in a positive mood, while patients suffering from depression show reduced levels of serotonin. As a result, the most modern medications to treat depression are serotonin reuptake inhibitors (SSRIs), which act to counteract the reduced levels of serotonin by increasing the amount of serotonin available to the neurons (Dfarhud et al., 2014). SSRIs are highly potent and specific antidepressants that bind and inhibit the SERT, preventing it from transporting serotonin back to the presynaptic neuron. This increases the availability of serotonin in the synapse and allows more serotine to bind to postsynaptic receptors. SSRIs can also inhibit the presynaptic autoreceptor, inhibiting the negative feedback so that serotonin continues to be released from the presynaptic cell, further increasing the amount of serotonin in the synapse (Mohammadzadeh et al., 2008).

However, increasing the use of antidepressants like these SSRIs can lead to serotonin toxicity, known as serotonin syndrome. This is a potentially life-threatening condition with side effects including euphoria, hyperreflexia (overactive muscle reflexes), and myoclonus (muscle twitches), as well as salivation, tremors and, potentially, death. These symptoms can appear in as little as an hour in acute cases. As new, more potent, antidepressants are developed, an increase in cases of serotonin syndrome has been reported. The severity of the condition depends on the dose of the antidepressant. The surest way to treat serotonin syndrome is to discontinue antidepressant use, though in many cases an adjustment of the dosage or a change of medication type can be sufficient (Mohammadzadeh et al., 2008).
Inactivation or Reuptake of Neurotransmitters
When neurotransmitters have crossed the synaptic cleft and delivered their message, they must be rapidly removed from the synaptic cleft. This mechanism is essential in order for synaptic signalling to happen efficiently, prevent diffusion, and for the synapse to remain sensitive to further signalling (Hyman, 2005; Robinson & Coyle, 1987). These neurotransmitters are removed from the synapse in a variety of neurotransmitter inactivation methods, for example, neurotransmitter-specific active transporters or enzymatic degradation (Robinson & Coyle, 1987). Classic neurotransmitters have a membrane reuptake mechanism. For example, amino acid neurotransmitters are taken back into the cell by highly specific, high-affinity transporters, while other neurotransmitters, like acetylcholine, can be first broken down by enzymes in the extracellular space before their substituents are reabsorbed (Todorov et al., 1997). This reuptake happens at both the cell and storage vesicle membrane. Transporters can quickly uptake and replace neurotransmitters at the site where they’re released so that axonal transport isn’t needed. Reuptake has only been discovered for classic neurotransmitters and hasn’t been shown to occur for neuropeptides (Hökfelt et al., 2018).

A less common system of neurotransmitter inactivation is enzymatic degradation. For example, acetylcholine is degraded by acetylcholinesterase, a highly active ectoenzyme found in cholinergic synapses. Enzymes work to break neurotransmitters down into their metabolites in the synaptic cleft. These metabolites can then be reabsorbed. Many neuropeptides are also degraded by enzymes. Neurotransmitter inactivation is essential to prevent a build-up of neurotransmitters, which would impede neuronal communication (Hyman, 2005).
Conclusion
Neurotransmitter signalling is essential for life. By exerting inhibitory or excitatory signals on neurons, neurotransmitters facilitate information to be broadcast from the brain to the periphery and back. This is an essential step in neural communication. With their vast array of functions, disruption of neurotransmitters or their targets can result in neurodegenerative and neurological disorders. Injury or disease can affect the synthesis of certain neurotransmitters and cause them to be under or over-produced. Depending on the neurotransmitter, this can have serious consequences. As the levels of neurotransmitters can directly influence neurodegenerative diseases like Parkinson’s, as well as conditions like depression, drugs to increase or regulate neurotransmitters are extremely promising for a future where these diseases are treatable. Neurotransmitter inactivation is essential to prevent a build-up of neurotransmitters, which would impede neuronal communication. Neurotransmitter reuptake and inactivator systems are essential to nervous system functionality (Hyman, 2005).
Bibliographical References
Ahlskog, J. E. (2007). Beating a dead horse: Dopamine and Parkinson disease. Neurology, 69(17), 1701–1711. https://doi.org/10.1212/01.wnl.0000296942.14309.4a
Avoli, M., & Krnjević, K. (2016). The long and winding road to gamma-amino-butyric acid as neurotransmitter. Canadian Journal of Neurological Sciences, 43(2), 219–226. https://doi.org/10.1017/cjn.2015.333
Bettler, B., & Mulle, C. (1995). AMPA and kainate receptors. Neuropharmacology, 34(2), 123–139. https://doi.org/10.1016/0028-3908(94)00141-e
Berke, J. D. (2018). What does dopamine mean? Nature Neuroscience, 21(6), 787–793. https://doi.org/10.1038/s41593-018-0152-y
Bloem, B. R., Okun, M. S., & Klein, C. (2021). Parkinson’s disease. The Lancet, 397(10291), 2284–2303. https://doi.org/10.1016/s0140-6736(21)00218-x
Brown, D. A. (2006). Acetylcholine. British Journal of Pharmacology, 147(S1), S120–S126. https://doi.org/10.1038/sj.bjp.0706474
Deiana, S., Platt, B., & Riedel, G. (2011). The cholinergic system and spatial learning. Behavioural Brain Research, 221(2), 389–411. https://doi.org/10.1016/j.bbr.2010.11.036
Dfarhud, D., Malmir, M., & Khanahmadi, M. (2014). Happiness & health: The biological factors- Systematic review article. Iranian Journal of Public Health 43(11), 1468–1477. https://pubmed.ncbi.nlm.nih.gov/26060713
Eccles, J. C., Fatt, P., & Koketsu, K. (1954). Cholinergic and inhibitory synapses in a pathway from motor-axon collaterals to motoneurones. The Journal of Physiology, 126(3), 524–562. https://doi.org/10.1113/jphysiol.1954.sp005226
Herlenius, E., & Lagercrantz, H. (2004). Development of neurotransmitter systems during critical periods. Experimental Neurology, 190, 8–21. https://doi.org/10.1016/j.expneurol.2004.03.027
Hökfelt, T., Barde, S., Xu, Z., Kuteeva, E., Rüegg, J., Maître, E. L., Risling, M., Kehr, J., Ihnatko, R., Theodorsson, E., Palkovits, M., Deakin, W. J., Bagdy, G., Juhasz, G., Prud’homme, H. J., Mechawar, N., Diaz-Heijtz, R., & Ögren, S. O. (2018). Neuropeptide and small transmitter coexistence: Fundamental studies and relevance to mental illness. Frontiers in Neural Circuits, 12. https://doi.org/10.3389/fncir.2018.00106
Hyman, S. E. (2005). Neurotransmitters. Current Biology, 15(5), R154–R158. https://doi.org/10.1016/j.cub.2005.02.037
Janowsky, D., El-Yousef, K., & Davis, J. (1974). Acetylcholine and Depression. Psychosomatic Medicine, 36(3), 248–257. https://journals.lww.com/psychosomaticmedicine/Abstract/1974/05000/Acetylcholine_and_Depression_.8.aspx
Kim, J. J., Gharpure, A., Teng, J., Zhuang, Y., Howard, R. J., Zhu, S., Noviello, C., Walsh, R. M., Lindahl, E., & Hibbs, R. (2020). Shared structural mechanisms of general anaesthetics and benzodiazepines. Nature, 585(7824), 303–308. https://doi.org/10.1038/s41586-020-2654-5
Latif, S., Jahangeer, M., Razia, D. M., Ashiq, M., Ghaffar, A., Akram, M., Allam, A. E., Bouyahya, A., Garipova, L., Shariati, M. A., Thiruvengadam, M., & Ansari, M. A. (2021). Dopamine in Parkinson’s disease. Clinica Chimica Acta, 522, 114–126. https://doi.org/10.1016/j.cca.2021.08.009
Li, Z. J., & Cho, C. H. (2011). Neurotransmitters, more than meets the eye-neurotransmitters and their perspectives in cancer development and therapy. European Journal of Pharmacology, 667(1–3), 17–22. https://doi.org/10.1016/j.ejphar.2011.05.077
Mattson, M. P., & Kater, S. B. (1989). Excitatory and inhibitory neurotransmitters in the generation and degeneration of hippocampal neuroarchitecture. Brain Research, 478(2), 337–348. https://doi.org/10.1016/0006-8993(89)91514-x
Mesulam, M. (2013). Cholinergic circuitry of the human nucleus basalis and its fate in Alzheimer’s disease. Journal of Comparative Neurology, 521(18), 4124–4144. https://doi.org/10.1002/cne.23415
Mohammadzadeh, L., Moses, L. L., & Gwaltney-Brant, S. M. (2008). Serotonin: A review. Journal of Veterinary Pharmacology and Therapeutics, 31(3), 187–199. https://doi.org/10.1111/j.1365-2885.2008.00944.x
Moore, R. Y., & Speh, J. C. (1993). GABA is the principal neurotransmitter of the circadian system. Neuroscience Letters, 150(1), 112–116. https://doi.org/10.1016/0304-3940(93)90120-a
Moret, C., & Briley, M. (2011). The importance of norepinephrine in depression. Neuropsychiatric Disease and Treatment, 9. https://doi.org/10.2147/ndt.s19619
Prescot, A. P., Becerra, L., Pendse, G., Tully, S., Jensen, E. S., Hargreaves, R., Renshaw, P. F., Burstein, R., & Borsook, D. (2009). Excitatory neurotransmitters in brain regions in interictal migraine patients. Molecular Pain, 5, 1744–34. https://doi.org/10.1186/1744-8069-5-34
Rizzi, G., & Tan, K. R. (2017). Dopamine and acetylcholine, a circuit point of view in Parkinson’s disease. Frontiers in Neural Circuits, 11. https://doi.org/10.3389/fncir.2017.00110
Robinson, M. B., & Coyle, J. T. (1987). Glutamate and related acidic excitatory neurotransmitters: From basic science to clinical application. The FASEB Journal, 1(6), 446–455. https://doi.org/10.1096/fasebj.1.6.2890549
Rommelfanger, K. S., & Weinshenker, D. (2007). Norepinephrine: The redheaded stepchild of Parkinson’s disease. Biochemical Pharmacology, 74(2), 177–190. https://doi.org/10.1016/j.bcp.2007.01.036
Silverberg, A. B., Shah, S. D., Haymond, M. W., & Cryer, P. E. (1978). Norepinephrine: Hormone and neurotransmitter in man. American Journal of Physiology-endocrinology and Metabolism, 234(3), E252. https://doi.org/10.1152/ajpendo.1978.234.3.e252
Todorov, L. D., Mihaylova-Todorova, S., Westfall, T. D., Sneddon, P., Kennedy, C., Bjur, R. A., & Westfall, D. P. (1997). Neuronal release of soluble nucleotidases and their role in neurotransmitter inactivation. Nature, 387(6628), 76–79. https://doi.org/10.1038/387076a0
Wallace, T. L., & Bertrand, D. (2013). Importance of the nicotinic acetylcholine receptor system in the prefrontal cortex. Biochemical Pharmacology, 85(12), 1713–1720. https://doi.org/10.1016/j.bcp.2013.04.001
Wenk, G. L. (1997). The nucleus basalis magnocellularis cholinergic system: One hundred years of progress. Neurobiology of Learning and Memory, 67(2), 85–95. https://doi.org/10.1006/nlme.1996.3757
Whiting, P. J. (2003). GABA-A receptor subtypes in the brain: A paradigm for CNS drug discovery? Drug Discovery Today, 8(10), 445–450. https://doi.org/10.1016/s1359-6446(03)02703-x
Visual Sources
Cover image: Unknown. (2020). Which neurotransmitter is involved in drug addiction? [Image]. Master Center. https://mastercenter.com/which-neurotransmitter-involved-drug-addiction/
Figure 1: Unknown. (n.d.). Synapse structure. [Image]. Michigan State University. https://openbooks.lib.msu.edu/neuroscience/chapter/synapse-structure/
Figure 2: Guy-Evans, O. (2023). Types of neurotransmitters. [Table]. Simply Psychology. https://www.simplypsychology.org/neurotransmitter.html
Figure 3: Guy-Evans, O. (2023). Neurotransmitters function. [Image]. Simply Psychology. https://www.simplypsychology.org/neurotransmitter.html
Figure 4: Unknown. (n.d.). Acetylcholine. [Image]. Health Jade. https://healthjade.net/acetylcholine/
Figure 5: Muramatsu, I., Uwada, J., Yoshiki, H., Sada, K., Lee, K. S., Yazawa, T., Tangiguchi, T., Nishio, M., Ishibashi, T., Masuoka, T. (2019). Cholinergic terminals. [Image]. Journal of Neurochemistry. https://onlinelibrary.wiley.com/doi/epdf/10.1111/jnc.14701
Figure 6: Takeda, S. (2019). Tau propagation as a diagnostic and therapeutic target for dementia: Potentials and unanswered questions. [Image]. Frontiers. https://www.frontiersin.org/articles/10.3389/fnins.2019.01274/full
Figure 7: Konkel, L. (2022). What is dopamine? [Image]. Everyday Health. https://www.everydayhealth.com/dopamine/
Figure 8: Tran N. H., Nguyen, J., Nolan, K., Park, H., Lam, S., Fattah, M., Page, J. C., Hang-Eun, J., Martin B.G. Jun, Lee, H., Kim, S., Shi, J., Lee, H. (2009). Implant to better track brain chemical gone rogue after neurotrauma. [Image]. Perdue University. https://sciencesources.eurekalert.org/news-releases/664064
Figure 9: Unknown. (2022). Noradrenaline or norepinephrine: Hormone or neurotransmitter. [Table]. Senesco. https://sanescohealth.com/blog/noradrenaline-or-norepinephrine-hormone-or-neurotransmitter/
Figure 10: Unknown. (2023). GABA Neurotransmitter. [Image]. GABA Neurotransmitter Collection. https://cpictures.homes/gaba-neurotransmitter
Figure 11: Unknown. (n.d.). Serotonin molecule. [Image]. iStockPhoto. https://www.istockphoto.com/photos/serotonin-molecule
Figure 12: Kaciroti, N. (n.d.). Mechanism of action of SSRIs. [Image]. Research Gate. https://www.researchgate.net/figure/Schematic-diagram-showing-mechanism-of-action-of-SSRIs-These-agents-block-the-reuptake_fig1_7730046
Figure 13: Unknown. (n.d.). Neurotransmitters. [Image]. Cleveland Clinic. https://my.clevelandclinic.org/health/articles/22513-neurotransmitters







Comments